Sparingly soluble in water. Suspended sodium hydrogen carbonate is removed from the carbonating tower and heated at 300oc to produce sodium carbonate.
Solvay Process For Producing Sodium Carbonate Current Technology
Reactions of sodium carbonate preparing urea and naoh reaction.
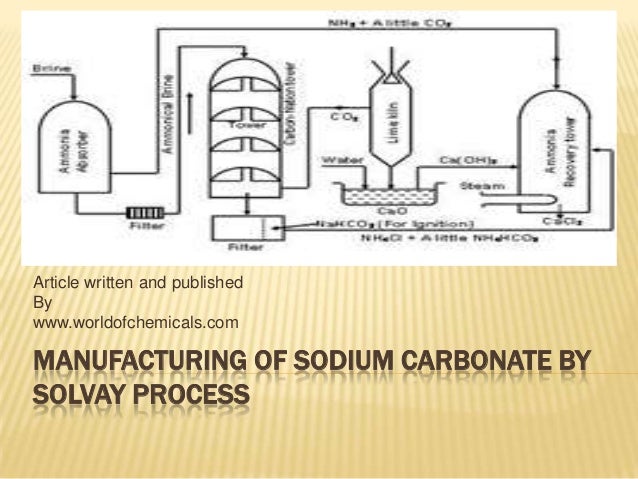
Sodium carbonate production process. Questions and answers of na 2 co 3 and solvay process is sodium carbonate acidic or basic. The french academy wanted to promote the production of much needed sodium carbonate from inexpensive sodium. The solvay process quickly came to dominate sodium carbonate production worldwide.
Since the 1860 s sodium carbonate has been produced using the solvay process. In 1791 the french chemist nicolas leblanc patented a process for producing sodium carbonate from salt sulfuric acid limestone and coal. Ammonia and sodium carbonate are given as products by the reaction of urea and naoh.
The solvay process is a continuous process using limestone caco 3 to produce carbon dioxide co 2 which reacts with ammonia nh 3 dissolved in brine. Although nahco 3 is produced in the solvnahco 3 is used in solvay process heating it to remove the ammonia it is contaminated with decomposes some nahco 3 so it is actually cheaper to react the finished na 2 co 3 product with co 2. Formation of sodium carbonate.
Sodium bicarbonate or baking soda nahco3. 2nahco 3 s na 2 co 3 s h 2 o g co 2 g ammonia recovery. Process for producing sodium carbonate with ammonia and or for producing refined sodium bicarbonate wherein.
Sodium bicarbonate baking soda sodium hydrogen carbonate is obtained as an intermediate product in solvay process for the manufacturing of sodium carbonate. Baking soda and fire extinguishers. Sodium carbonate dissociates completely in the water to na ion co.
Cao is formed as a by product of the thermal decomposition of limestone in the lime kiln. Sodium carbonate is a basic compound. A low co 2 content gas generated by a unit for producing sodium carbonate with ammonia.
Manufacturing sodium carbonate by the solvay process an account of the solvay process. Sodium carbonate na 2 co 3 has a number of uses but its most common use is in the production of glass. This resource is part of the sodium carbonate a versatile material resources collection which presents learning material based on the manufacture and uses of sodium carbonate made by the solvay ammonia soda process.
By 1900 90 of sodium carbonate was produced by the solvay process and the last leblanc process plant closed in the early 1920s. Removing sulfur dioxide so 2 from flue gases in power stations. Properties of sodium bicarbonate.